Day 2 :
Keynote Forum
Jean Francois Silvain
ICMCB-CNRS, France
Keynote: Physical properties of metal-matrix composite materials
Time : 10:00-10:35

Biography:
Jean-Francois Silvain is a Senior Researcher at ICMCB-CNRS and an Adjunct Professor of Engineering at UNL-Lincoln, USA. He has obtained his Bachelor’s degree at Poitiers University in 1980 and PhD degree at Poitiers University in 1984, both in Material Science. He was a CNRS Research Fellow at the University of Nancy, France from 1987 to 2002. He has joined the ICMCB-CNRS in 1992. He has over 30 years of experience in composite materials processing and characterization at micro/nano scales. His main field of interest is the processing and the characterization of ceramic and inorganic multi materials ranging from metal matrix composite to functionally graded materials with the aim to develop materials with adaptive physical and/or mechanical properties.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
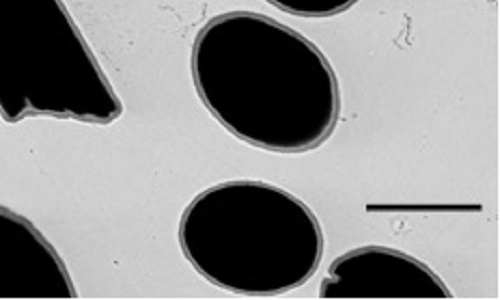
Both high thermal conductivities and low thermal expansion coefficients (CTE) are required for heat-sink materials as they promote rapid heat dissipation and tolerate thermo-mechanical strains upon thermal cycling. Currently, Cu or Al heat sinks are being used. However, they are not suitable due to the large CTE mismatch with the ceramic and silicon parts in the components. To overcome this issue, we proposed to replace the Cu and Al heat sinks by metal matrix composites (MMCs), more particularly Al and Cu matrix composites reinforced with carbon. The properties in MMCs is often compromised by the absence of effective interfaces, especially in non-reactive systems such as Al(Al2O3)/C and Cu/C. However, for a thermally efficient assembly, the interface should allow proper transfer of thermo-mechanical loads between the materials, which is only possible in the presence of chemical bonding. Ex-situ and in-situ methods can be used to form interfacial metal-matrix zones with optimized physical properties. An ex-situ method developed in this study is correlated with the synthesis of a hybrid TiOx/TiC coating on carbon fibers (CFs) using molten salt synthesis. The molten salt synthesis method is a facile and efficient way for the synthesis of transition metal carbides at low temperatures in a relatively short time. The in-situ method is linked with the synthesis of composite materials by alloying the matrix with carbide forming elements which has been investigated using a well-known process used for Al-based composites. The solid-liquid coexistence allows the formation of a liquid phase which enhances the reactivity between the carbide forming element and the carbon reinforcement. For both ex-situ and in-situ MMC composite materials, the fabrication conditions will be correlated with the microstructures of the interfacial zones and the physical properties of the MMC materials.
In situ formation of TiC interfacial zone between carbon fibers and copper matrix (bar equal 10 microns).
- Materials Science and Chemistry | Materials Science and Engineering | Materials Chemistry in Developing Areas | Analytical Techniques and Instrumentation in Materials Chemistry | Polymeric Materials
Location: St. Gallen II

Chair
Debajyoti Ghoshal
Jadavpur University, India
Session Introduction
Thieo E Hogen Esch
University of Southern California, USA
Title: Nano-scale membranes based on polystyrene sulfonic acid blends
Time : 12:10-12:30

Biography:
Thieo E Hogen Esch has completed his PhD at the University of Leiden, Netherlands. Since then he has carried out research in the areas of physical organic chemistry and in the stereochemistry of anionic vinyl polymerization, the synthesis of block copolymers by anionic and free radical polymerizations at the University of Florida and since 1988 at the University of Southern California (USC). He has pioneered the synthesis and properties of “perfluorocarbon functionalized polymers” a topic that remains current. During the last few years his research has also included the synthesis and properties of thermodynamically unstable polymer blends of “camouflage mediated” homogeneous hydrophobic-hydrophilic type membrane.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
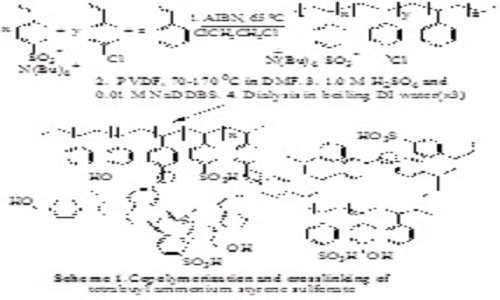
Flexible, strong, colorless and transparent polyvinylidenefluoride-olystyrenesulfonic acid (PVDF-PSSA) proton exchange membranes1-3 were synthesized by first mixing DMF solutions of PVDF and tetrabutylammonium polystyrenesulfonate (PTBASS) terpolymer having smaller (<20%) mole fractions of styrene (S) and 4-chloromethylstyrene (CMS). The tetrabutylammonium styrene (BASS) sulfonate precursor monomer was synthesized through simple ion exchange of sodium styrene sulfonate and tetrabutylammonium-chloride or bromide. The PBASS terpolymer precursor was synthesized through a conventional radical copolymerization of BASS, styrene and CMS. The PVDF-PBASS blends are formed by dissolution of the two polymers in DMF, followed by evaporation and thermal cross-linking through annealing at about 170°C. The EAS type crosslinking appears to involve the formation of TBA benzyl sulfonates that may directly benzylate the S and CMS units of the terpolymer. The PVDF-PBASS blends are optically transparent above about 165°C and remain so after rapid cooling followed by ion exchange mediated by a mixture of 1.0 M H2SO4 and surfactants and repeated aqueous dialysis giving low (<40 wt%) water content PVDF-PSSA membranes.4 Transmission electron microscopy confirms the presence of small (≥5 nm) PSSA domains the size and nature of which is still unknown. The water uptake, proton conductivity, ion exchange capacity (IEC), and methanol permeability of the membranes are tunable through variation of the PVDF/PSSA content and the sulfonic acid content of the terpolymer. At higher PSSA contents (>20 wt.%) the membranes were shown to have proton conductivities comparable or higher than Nafion-117 but significantly lower methanol permeabilities. These low cost, environmentally appealing and conveniently accessible membranes can be used in direct methanol fuel cells (DMFCs) and other applications.
Vincent Ball
University of Strasbourg, France
Title: From prebiotic chemistry to materials science: Deposition of aminomalononitrile based films
Time : 12:30-12:50

Biography:
Vincent Ball has studied Physics and Physical Chemistry at the Université Louis Pasteur (ULP), in Strasbourg, where he got his PhD in 1996. After a Postdoc at the Biozentrum Basel from 1996 to 1997, he was appointed as the Assistant Professor in Analytical Chemistry at ULP and Professor in Chemical Engineering in 2005. His research interests focus on thermodynamics and kinetics of self-assembly at Interfaces, biomimetic chemistry and the design of biomaterials with designed electrochemical and mechanical properties. He published a monography “Self-assembly processes at Interfaces-Multiscale phenomena” at Academic Press) in 2017. He was an Invited Researcher at the Michigan University in 2007 as a Fulbright fellow as well as in CSIRO-Clayton in 2018.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
Surface functionalization with strongly adhesive and easy to functionalize films has remained highly materials specific for a long time. But bio inspiration from the mussel which is able to adhere to almost all known materials under wet conditions has allowed the development of polydopamine films from the oxidation of dopamine. Other molecules seem promising in this perspective: inspiration from prebiotic chemistry and from the famous experience realized by Prof. Miller which allowed it to demonstrate that strongly adhesive and biocompatible films can be deposited at solid-liquid interfaces from aminomalonitrile solutions. In this presentation it will be demonstrated that similar aminomalononitrile based films can be obtained by electropolymerization. The mechanism of the film deposition and their morphology (Figure) will also be described. In particular we will show that films can also be deposited at liquid-air interfaces but having morphology and a composition different from that deposited at solid-liquid interfaces.
A: water contact angles of AMN based films as a function of the deposition time, and B, C: film morphology after 1 and 19 h as characterized by SEM (B) and AFM (C).
Lunch Break 13:00-13:50 @ Foyer
Saikat Kumar Seth
Jadavpur University, India
Title: Salt-bridge (SB) interaction at work in building extended supramolecular networks: Experimental and theoretical study
Time : 13:50-14:10

Biography:
Saikat Kumar Seth is an Assistant Professor of Physics at Jadavpur University, Kolkata, India. He was awarded PhD at Indian Association for the Cultivation of Science (IACS), Kolkata in 2012 and Postdoctoral at Universitat de Illes Balears, Palma, SPAIN. His area of research is X-ray Crystallography and Crystal Engineering. The focus of his research is to explore weak noncovalent interactions in the context of Crystal Engineering along with high-performance computational studies. He had published 72-research articles in various peer-reviewed journals, edited one book (National level) and contributed a book chapter in Royal Society of Chemistry (RSC). He got various awards like Young Scientist, Best Poster, Best Presentation, and Research Excellence, Outstanding Reviewer award from 4 Elsevier Journals and Publons Peer Review Award in global peer review.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
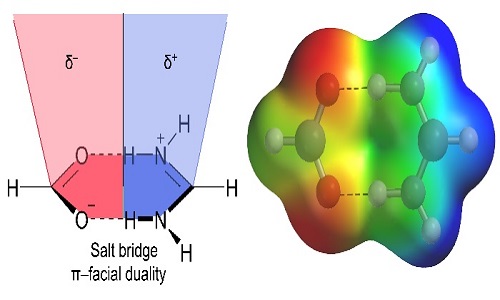
A series of pyridinium-carboxylate salts were designed prepared and structurally characterized to explore the importance of salt-bridge (SB) interactions in building self-assembled structures. We present a comprehensive analysis of the SB interaction in crystal structures of 4, 4′-Oxybis (benzoic acid) with substituted aminopyridines where the SBs displays extremely well defined geometric preferences. In the solid-state, compound exhibit lone-pair (l.p) ··· (SB)/(SB)···π+ assemblies while compound shows C‒H···(SB)/(SB)···π network. Interestingly, compound exhibit two distinct networks π+···(SB)/(SB)···(SB)/(SB)···π+ and C‒H···(SB)/(SB)···H‒C. These unexplored extended supramolecular networks are evident and explored for the first time in crystal structures. Our study also describes the duality of the salt bridge in establishing π-facial interactions with electron rich and/or electron poor moieties depending on the positive or negative nature of the SB counterpart that interacts with the SB surface. We describe the energetic and geometric features of salt-bridge interaction and explore its impact on the resultant supramolecular organization using high-level theoretical DFT-D3 calculations. The theoretical study combines the energetic features of the noncovalent forces that participate in the extended network and the characterization of the diverse interactions by means of Bader’s theory of ‘‘atoms in molecules’’.
Partha Pratim Ray
Jadavpur University, India
Title: Piezo response of defects mediated Methyl Ammonium Lead Iodide (MAPbI3)
Time : 14:10-14:30

Biography:
Partha Pratim Ray is working as an Associate Professor in the Department of Physics, at Jadavpur University, Kolkata, India. He has done his PhD and Postdoctoral research work on thin film silicon based solar cell. He is working on synthesis and application of different nanostructured materials in energy harvesting devices. He has studied the effect of different synthesis methods on charge transport properties which determines device performance. He is also working on metal organic framework based thin film photosensitive Schottky diode.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
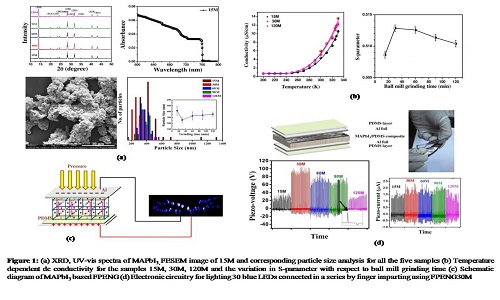
Formation of lattice defects (point defects/dislocations/grain boundaries) is an unavoidable phenomenon which is associated with almost all synthesis procedures following fast formation kinetics. Therefore a polycrystalline thin film of hybrid perovskite developed for a solar cell invariably contains lattice defects. Similarly in a systematic fashion using ball mill grinding technique we have synthesized perovskite material, Methylammonium lead iodide (MAPbI3) with different degrees of crystal defects as probed by positron annihilation spectroscopy (PAS) and utilized to fabricate flexible piezoelectric nanogenerators (FPENGs). Five sets of MAPbI3 samples are prepared by ball mill grinding procedure with different grinding time (15m, 30m, 60m, 90m and 120m). The formation and morphology of MAPbI3 is confirmed from their powder XRD pattern and field emission scanning electron microscopy (FESEM) images. The optical band gaps (1.63 eV) of all the samples are calculated from their absorption onset at 760 nm. The x-ray diffraction pattern suggests the formation of tetragonal crystal phase. We have demonstrated that at room temperature the lattice defects play the pivotal role in governing the ionic polarization from temperature dependent dc conductivity measurement and establish one-to-one correlation with the lattice defects as probed by PAS, which in principle governs the piezo-effect in MAPbI3. Here, we have shown that lattice defect mediated ionic polarization significantly changes VOC, but ISC remains almost same for all the samples as ISC has its origin on the value of piezoelectric constant and elastic modulus of the material. The best device performance is exhibited by maximum defect containing sample (30m) having significant amount of Pb2+ defects. A device fabricated with 5 wt % PDMS composite produces piezo-voltage (>100V) with a maximum power density of 0.3 mW/cm3 and can illuminate commercially available 30 blue light emitting diodes.
- Nanomaterials | Inorganic Materials Chemistry | Organic Materials Chemistry | Materials Chemistry and Physics | Science and Technology of Advanced Materials
Location: St. Gallen II

Chair
Jean-Francois Silvain
ICMCB-CNRS, France
Session Introduction
Tofik M Nagiev
Nagiev Institute of Catalysis and Inorganic Chemistry, Azerbaijan
Title: Nitrogen fixation at conjugated oxidation by hydrogen peroxide
Time : 12:05-12:25

Biography:
Tofik M Nagiev is a Vice-president of Azerbaijan National Academy of Sciences, Director of Research Center of “Azerbaijan National Encyclopedia” and Department chief of Nagiev Institute of Catalysis and inorganic chemistry of ANAS. He is the Professor of the department of the Physical and Colloid Chemistry of Baku State University.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
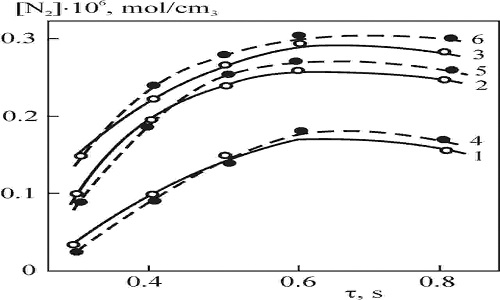
The problem of nitrogen fixation is one of the most important scientific and technical tasks. Nitrogen and nitrogen compound making industry is one of the leading branches of the modern chemical industry. The state of the art of it is the main factor affecting supply of nitrogenous fertilizers to agriculture and of various nitrogen-containing substances to industry. Fig 1 shows dependence of molecular nitrogen conversion on its volume rate in the temperature range of 773-873 K. It is clearly observed that the quantity of fixed nitrogen per injected molecular nitrogen increases to some extent with the amount of injected molecular nitrogen. A further increase of raw material rate does not change the level of nitrogen fixation. Despite shorter contact time, the increase of N2 volume rate from 1 to 4 l/h intensifies the reaction. The aqueous hydroperoxide rate is constant for all nitrogen rates. However, at nitrogen rate of 4-5 l/h the fixed nitrogen yield is altered. Thus, chemical induction is the main factor promoting N2 fixation in N2-H2O2-H2O system. It manifests itself owing to coupling of H2O2 dissociation reactions, which generate the intermediate-HO2 radical-to the system. This intermediate transfers the induction action of the primary reaction to N2 oxidation process. In this case, H2O2 is injected in amounts much greater than demanded by N2 oxidation, because the main requirement for effective chemical coupling is the presence of HO2 in high concentration. To conclude the discussion, it should be noted that oxidative fixation of molecular nitrogen with hydrogen peroxide is rather simple for process engineering design, usually proceeds under homogeneous conditions without any catalyst under atmospheric pressure, and produces high yield of fixed nitrogen.
Kinetics of nitrogen fixation with hydrogen peroxide: molar ratio N2:30% H2O2 = 1:1.6; 1 – 3 – T = 773, 823 and 873 K, respectively; 4 – 6 – theoretical curves at these temperatures
Alejandro Perez Florez
Pontificia Universidad Javeriana, Colombia
Title: A novel and facile route for CuMn-MgAl mixed oxides catalysts to catalytic degradation of violet crystal in water
Time : 12:25-12:45

Biography:
Alejandro Perez is currently working at the Pontificia Universidad Javeriana in Bogota, Colombia as a Teacher and Researcher in the line of environmental technology and materials, where they have developed a wide range of solids for water treatment. He has had the opportunity to learn and deepen in spectroscopic, texture and microscopic techniques for the characterization of materials. Also, in chromatographic techniques for monitoring the reactions evaluated.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
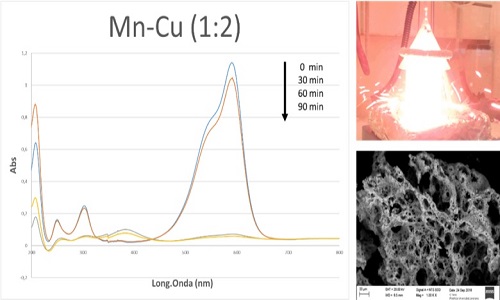
Currently the textile industry, educational centers and laboratories have a pollution problem due to the irresponsible discharge of dye wastewater into the waterways, which is generating a great impact on the quality and aesthetics of water resources. Recently, the use of mixed oxides has gained a great deal of interest in the advanced oxidation process (AOP) for the treatment of waste water of dyes due to its good degradation efficiency, low toxicity and physical and chemical properties. However, still using extreme conditions to remove contaminants, limits the use of these techniques for future industrial use. In this work the degradation of wastewater containing 50 mg/L violet crystal (VC) by air oxidation in a continuous flow trickle-bed reactor over CuxMnyMgAl catalyst obtained by auto combustion method were studied. It was found the decolorization efficiency and the chemical oxygen demand (COD) removal of VC reached above 92% and 100%, respectively, within 60 min at room temperature and atmospheric pressure. The total organic carbon (TOC) decreased 76.1%. The obtained samples were characterized by powder X-ray diffraction, fourier transform infrared, atomic absorption spectrometry, scanning electron microscope, N2 adsorption and temperature programmed reduction with H2. The results show that the activity on violet crystal oxidation was significantly influenced by the preparation method of the mixed oxides. The auto combustion method provides better catalysts because the redox properties improve and the specific area increase. The most effective catalyst was Cu2Mn1MgAl, with 100% of decolorization in 1 hour of the reaction. A real textile wastewater was also successfully treated with this catalyst. The monitoring by CG-MS allows recognizing the mineralization of the VC to CO2, H2O and compounds with shorter chains less toxic than the initial dye.
Debasish Biswas
Jadavpur University, India
Title: Observing giant room temperature magneto dielectric response in silica-based oxide nano glass
Time : 13:30-13:50

Biography:
Debasish Biswas has obtained his PhD in Experimental Physics at the University of Calcutta in 1999; Postdoctoral research work on ion trap spectroscopy in Penning trap. He has published in areas relating to spectroscopic study of atmospheric molecules and trace gases. Currently, he is a Professor in the Department of Physics at Jadavpur University, Kolkata, India and his primary role is of an Educator. His current research interest includes nanomaterials, space physics and atom optics.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
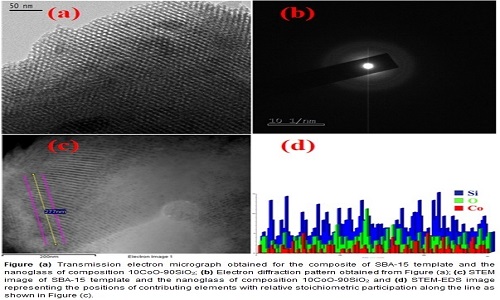
Nano dimensional silica-based glasses of compositions xCoO-(1-x)SiO2 with x having values 10 and 30 were synthesized by a sol-gel method within the nanopores of mesoporous template. X-ray photoelectron spectroscopic (XPS) analysis showed the presence of Co2+ and Co3+ species in the glasses causing electronic conduction in this amorphous system. At room temperature the resistivity values exhibited by the nano glasses were nearly three orders of magnitude lower than those of the corresponding bulk glasses. The resistivity data for the nano glasses on analysis confirmed the conduction to arise due to small polaron hopping between the localized states represented by those ions. Typical values of inter-site separation distance extracted from the said analysis were found to be ~7 Å. The values of magneto dielectric parameter for the different nanocomposites were large with the highest value found to be in the range 500% to 45% for the frequencies 1 kHz and 1 MHz respectively for a nanocomposite with glass composition of 30CoO-70SiO2. The results were then fitted to Catalon’s model based on two dielectrics in series with different resistivity values. The satisfactory fit of the measured data to the theoretical model based on a negative magneto resistance of the nano glasses indicate that an enhancement of spin polarized electron hopping caused this effect. The magnetization change as a function of the applied magnetic field was calculated for the nanocomposites. The super exchange interaction between Co2+ and Co3+ ions through the intervening oxygen ions gave rise to the ferromagnetic like behaviour of the samples. In future these materials may be used as good magnetic sensors.
Jean Francois Silvain
ICMCB-CNRS, France
Title: Thermal and thermomechanical properties of graphite flakes and carbon fibers reinforced aluminum matrix composites
Time : 13:50-14:10

Biography:
Jean-Francois Silvain is a Senior Researcher at ICMCB-CNRS and an Adjunct Professor of Engineering at UNL-Lincoln, USA. He has obtained his Bachelor’s degree at Poitiers University in 1980 and PhD degree at Poitiers University in 1984, both in Material Science. He was a CNRS Research Fellow at the University of Nancy, France from 1987 to 2002. He has joined the ICMCB-CNRS in 1992. He has over 30 years of experience in composite materials processing and characterization at micro/nano scales. His main field of interest is the processing and the characterization of ceramic and inorganic multi materials ranging from metal matrix composite to functionally graded materials with the aim to develop materials with adaptive physical and/or mechanical properties.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
Today, the microelectronics industry uses higher functioning frequencies in commercialized components. These frequencies result in higher functioning temperatures and, therefore, limit a component’s integrity and lifetime. Until now, heat-sink materials were composed of metals which exhibit high thermal conductivities (TC). However, these metals often induce large coefficient of thermal expansion (CTE) mismatches between the heat sink and the nonmetallic components of the device. Such differences in CTEs cause thermomechanical stresses at the interfaces and result in component failure after several on/off cycles. To overcome this issue, one solution is to replace the metallic heat sink materials by a metal matrix composites (MMCs), specifically, carbon-reinforced aluminum matrix (Al/C) which exhibit optimized thermomechanical properties. Moreover, due to his low density, 2.7 g/cm3, Al has a high specific thermal conductivity (TC divided by density) and low cost which are great advantages in terms of the fabrication of mobile electronic devices for automobile or aeronautic industries. Carbon fibers (CF) and graphite flakes (GF) were preferred to diamond particles because of their low coefficient of thermal expansion (CTE), high thermal conductivity (TC), and a good machinability. These reinforcements will allow developing composite materials that fulfill the requirements related to the field of power electronics. However, the anisotropic thermal properties of these reinforcements may generate a strong influence of the reinforcement orientation on the macroscopic thermal properties of the MMC. Moreover, the oxide layer on Al particles inhibits Al-C reactivity. Therefore, the areas of research discussed in this work are: Orientation optimization of anisotropic reinforcements by a microstructural design Development of composite materials with mixed reinforcement (GF+FC) to evaluate the influence of the combination of the two reinforcements on the thermomechanical properties; Elaboration of composite materials by liquid phase sintering in order to optimize the interfacial properties and densification of composite materials with high carbon content.
Debajyoti Ghoshal
Jadavpur University, India
Title: Transformation directed functionality in dynamic metal-organic framework based materials
Time : 14:10-14:30

Biography:
Debajyoti Ghoshal has completed his Doctoral studies at Indian Association for the Cultivation of Science, Kolkata and got his PhD at Jadavpur University, India in 2005. After that he has worked at the University of Gottingen, Germany as an Alexander von Humboldt fellow, to purse his Postdoctoral research. Currently, he is an Associate Professor in the Department of Chemistry at Jadavpur University and his current research interest includes functional metal organic frameworks (MOF) with a special emphasis to the selective gas storage, conducting MOF and phase transformation in dynamic MOF. He has published more than 75 papers in peer reviewed journals which has received 1780 citation with h index of 26.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
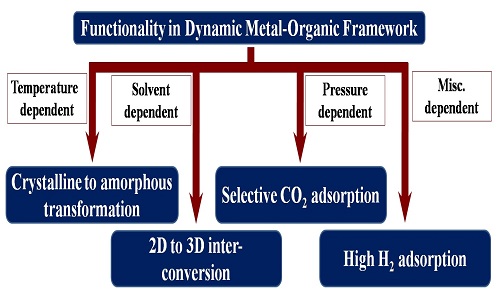
Dynamic metal organic frameworks (MOFs) have become a very promising field in contemporary research not only due to their study in understanding structural features but also for their potential applications in tailoring better functionality. The transformation of phase which is the fundamental thing of such structural dynamicity can be initiated by various external stimuli like heat, light, pressure, solvent etc. This dynamism in the structure of MOF based material can proceeds through physical as well as chemical changes. Interestingly, it has been noted that for such dynamic MOFs, the transformed phase may sometime display stepwise gas and/or solvent adsorption and also show selective gas adsorption. These functionalities in dynamic MOFs might be found pretty useful for gas and/or solvent separation. Moreover, as the change of phase are generally taken place by some external stimuli, monitoring of the change in the structures of dynamic MOF, can be used as a sensor for the responsive stimuli both as qualitative (molecular recognition and/or sensing) as well as quantitative (impurity measurement) way. In many of our recent work, it has been observed that in the study of flexible MOFs with their structural dynamicity most of them show reversible crystalline-to-crystalline transformation whereas in some cases rare reversible crystalline-to-amorphous transformation has also been observed. In every case, various external stimuli like heat, pressure, solvents etc, individually or cooperatively facilitate the transformations to another phase. There are several interesting properties have been observed for these transformed products. For example, one pair of MOFs show interesting solvent mediated inter-conversion from 2D to 3D framework and this change in dimension affects their adsorption properties too. In another endeavour, one pair of nitro functionalized MOFs exhibit selective CO2 adsorption after the transformation of phase. A polycatenated framework also shows a temperature as well as pressure mediated structural transformation; where the transformed phases are capable to show remarkable H2 uptake.
Tsan Yao Chen
National Tsing Hua University, Taiwan
Title: X-ray absorption spectroscopy and in-operando neutron diffraction studies on local structure fading induced irreversibility in a 18650 cell with P2-Na2/3Fe1/3Mn2/3O2 cathode in long cycle test
Time : 14:30-14:50

Biography:
Tsan Yao Chen is currently an Associate Professor at Department of Engineering and System Science, National Tsing Hua University. With more than 10 years of experience in Materials Characterization and 6 years in electronic device (MEMS and IC), failure analysis of multiple executives by Synchrotron Light Source Techniques (at NSRRC, Taiwan). He conduct fundamental materials development in green energy applications including solar cell, fuel cell, and CO2 conversion, water sensing with worldwide collaboration at National rank research team in Russia, USA, Italy, Japan (SPring-8), and UK. He is hosting research projects from academic and industrial funds on physical chemistry researches and environment sensing technologies.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
Cathode materials of P2-Na2/3[Fe1/3Mn2/3] O2 (NFMO) phase nanoparticle with a maximum capacity of ~148 mAh in a sodium ion battery was synthesized by a solid-state annealing method. By cross-referencing results of focused-ion beam (FIB) section scanning electron microscopy, ex-situ X-ray absorption spectroscopy (XAS), X-ray photoemission (XPS) depth profiling, and in-operando neutron diffraction, we found that Na ion intercalation and extraction distort the local structure in NFMO crystal, resulting in irreversibility of the sodium ion battery (SIB). This reaction pathway is controlled by the transformation kinetics of the Fe sites from octahedral (Oh) to tetragonal (Td) in the charge and discharge processes. For a SIB operated at 2.0 to 3.8V, steady kinetics between the Na intercalation and chemical state evolution on the Fe sites enable the homogeneous restructuring in both local and global regimes in NFMO crystal. For a SIB operated at 2.0 to 4.5V, substantially higher kinetics in the Fe chemical state evolution induces a dramatic lattice expansion. This expansion cracks the interface between the P2 and Na intercalated regions, thereby causing substantial irreversibility of NFMO in a SIB.
Networking Lunch 12:30-13:30 @ Foyer of St. Gallen II
- Poster Presentations
Location: Foyer
Session Introduction
Sol M Mejia
Pontificia Universidad Javeriana, Colombia
Title: Selective adsorption of mercury (Hg2+) on a metal-organic framework obtained from aluminium and mercaptosuccinic acid

Biography:
Sol M Mejia is an Assistant Professor at the Chemistry Department of the Pontificia Universidad Javeriana in Colombia since 2014. She received her PhD from the Universidad de Antioquia in 2011. She did two postdoctoral researches, the first one at the University of Adelaide in Australia and the second one at the Universidad Autónoma Metropolitana in Mexico. She is the leader of the Computational Chemistry research line. Her major areas of interest are radical organic reactions, metal clusters, metal organic frameworks, and weak interactions (hydrogen bonds) as basic chemistry with possible applications in chemistry catalysis, biologic activity, new materials, and the like.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
Abstract:
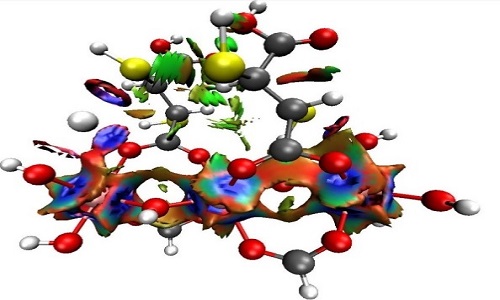
Water polluted with mercury represents a risk to the health of human beings and, in general, to all living beings that depend directly on these environments. It is then necessary to think about methods of capture and identification of chemicals species of mercury in water bodies. In this sense, the metalorganic frameworks (MOFs), hybrid crystalline materials, have a high potential to be used in the remediation of water contaminated with mercury. Through electronic structure calculations with density functional theory (DFT), the mechanisms of the interaction between Hg2+ and organic ligands were evaluated. These ligands were derived from fumaric acid when functionalized with groups -SH, - SCH3, -NH2, or -PH2. The interaction energy analysis, as well as the global reactivity indexes, suggest that the thiol group is the most selective to mercury compared to other water constituent metals such as calcium and lead (-56.40 kcal/mol, -48.99 kcal/mol, and -39.91 kcal/mol, respectively). This adsorptive capacity of the organic ligand was maintained by being part of a representative structure of the selected MOF. Through the analysis of charge transfer and non-covalent interactions index, it was verified that this adsorption was mediated by the tricoordination of mercury involving two oxygen atoms and the sulfur of the thiol group; these interactions are strong, mainly electrostatic, and not dispersive (see Figure 1). Taking as a basis the computational results, the Al-mercaptosuccinate MOF was synthesized and characterized. Its removal efficiency evidenced the high adsorptive capacity of this framework, reaching Hg2+ removal ratios of 99% at a concentration of the metal in aqueous solution of 10 μg/mL, in the same way this MOF shows to be selective against mercury in presence of other metals.
Isosurfaces obtained from the Non-Covalent Interactions (NCI) index at the MOF---Hg2+complex
Urs K Fischer
Ivoclar Vivadent AG, Liechtenstein
Title: Photoinitiating efficiency of visible light initiators based on tetraacyl derivatives of germanium and tin

Biography:
Urs K Fischer has his expertise in evaluation in research and development in the field of dental materials. His main topics are material characterization of new monomers, photoinitiators, and composites for dental applications.
To present & exhibit your MATERIALS @ our upcoming series PS: Materials Conferences | Materials Chemistry Conferences | Materials Chemistry 2020
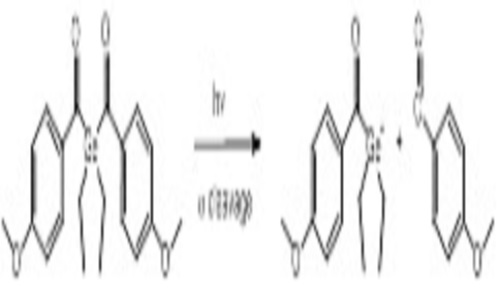
Abstract:
For several years, bisacylgermanium derivatives have been known to be very potent monomolecular initiators for photopolymerization induced by visible light (VL).[1] In particular, bis-(4-methoxybenzoyl) diethylgermane (Ivocerin®) exhibits an outstanding performance as amine-free Norrish type I photoinitiator (PI) for polymerization of dimethacrylate resins in dental materials and dimethacrylate-based composites, initialized by blue LED curing lights. Ivocerin® is characterized by strong absorption in the blue region of the VL spectrum, good solubility in and high PI reactivity towards dimethacrylate resins, good storage stability and excellent bleaching behavior. Recently, a very efficient synthetic method was developed, which allows the preparation of tetraacylgermanium derivatives according to a facile procedure in good yields. Based on the same synthetic procedure, the tin analogue tetrakis (2, 4, 6-trimethylbenzoyl) stannane is accessible, which displays a significant VL absorption beyond 500 nm.