Sol M Mejia
Pontificia Universidad Javeriana, Colombia
Title: Selective adsorption of mercury (Hg2+) on a metal-organic framework obtained from aluminium and mercaptosuccinic acid
Biography
Biography: Sol M Mejia
Abstract
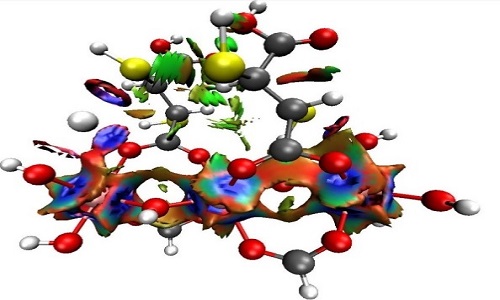
Water polluted with mercury represents a risk to the health of human beings and, in general, to all living beings that depend directly on these environments. It is then necessary to think about methods of capture and identification of chemicals species of mercury in water bodies. In this sense, the metalorganic frameworks (MOFs), hybrid crystalline materials, have a high potential to be used in the remediation of water contaminated with mercury. Through electronic structure calculations with density functional theory (DFT), the mechanisms of the interaction between Hg2+ and organic ligands were evaluated. These ligands were derived from fumaric acid when functionalized with groups -SH, - SCH3, -NH2, or -PH2. The interaction energy analysis, as well as the global reactivity indexes, suggest that the thiol group is the most selective to mercury compared to other water constituent metals such as calcium and lead (-56.40 kcal/mol, -48.99 kcal/mol, and -39.91 kcal/mol, respectively). This adsorptive capacity of the organic ligand was maintained by being part of a representative structure of the selected MOF. Through the analysis of charge transfer and non-covalent interactions index, it was verified that this adsorption was mediated by the tricoordination of mercury involving two oxygen atoms and the sulfur of the thiol group; these interactions are strong, mainly electrostatic, and not dispersive (see Figure 1). Taking as a basis the computational results, the Al-mercaptosuccinate MOF was synthesized and characterized. Its removal efficiency evidenced the high adsorptive capacity of this framework, reaching Hg2+ removal ratios of 99% at a concentration of the metal in aqueous solution of 10 μg/mL, in the same way this MOF shows to be selective against mercury in presence of other metals.
Isosurfaces obtained from the Non-Covalent Interactions (NCI) index at the MOF---Hg2+complex