Alejandro Perez Florez
Pontificia Universidad Javeriana, Colombia
Title: A novel and facile route for CuMn-MgAl mixed oxides catalysts to catalytic degradation of violet crystal in water
Biography
Biography: Alejandro Perez Florez
Abstract
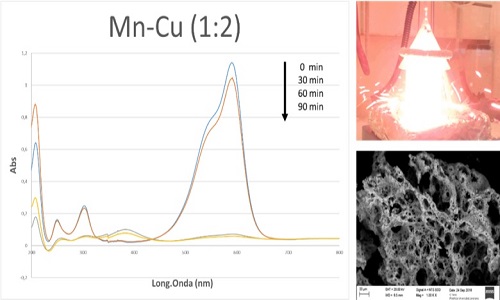
Currently the textile industry, educational centers and laboratories have a pollution problem due to the irresponsible discharge of dye wastewater into the waterways, which is generating a great impact on the quality and aesthetics of water resources. Recently, the use of mixed oxides has gained a great deal of interest in the advanced oxidation process (AOP) for the treatment of waste water of dyes due to its good degradation efficiency, low toxicity and physical and chemical properties. However, still using extreme conditions to remove contaminants, limits the use of these techniques for future industrial use. In this work the degradation of wastewater containing 50 mg/L violet crystal (VC) by air oxidation in a continuous flow trickle-bed reactor over CuxMnyMgAl catalyst obtained by auto combustion method were studied. It was found the decolorization efficiency and the chemical oxygen demand (COD) removal of VC reached above 92% and 100%, respectively, within 60 min at room temperature and atmospheric pressure. The total organic carbon (TOC) decreased 76.1%. The obtained samples were characterized by powder X-ray diffraction, fourier transform infrared, atomic absorption spectrometry, scanning electron microscope, N2 adsorption and temperature programmed reduction with H2. The results show that the activity on violet crystal oxidation was significantly influenced by the preparation method of the mixed oxides. The auto combustion method provides better catalysts because the redox properties improve and the specific area increase. The most effective catalyst was Cu2Mn1MgAl, with 100% of decolorization in 1 hour of the reaction. A real textile wastewater was also successfully treated with this catalyst. The monitoring by CG-MS allows recognizing the mineralization of the VC to CO2, H2O and compounds with shorter chains less toxic than the initial dye.