Tofik M Nagiev
Nagiev Institute of Catalysis and Inorganic Chemistry, Azerbaijan
Title: Nitrogen fixation at conjugated oxidation by hydrogen peroxide
Biography
Biography: Tofik M Nagiev
Abstract
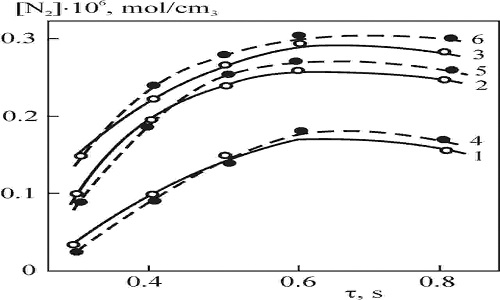
The problem of nitrogen fixation is one of the most important scientific and technical tasks. Nitrogen and nitrogen compound making industry is one of the leading branches of the modern chemical industry. The state of the art of it is the main factor affecting supply of nitrogenous fertilizers to agriculture and of various nitrogen-containing substances to industry. Fig 1 shows dependence of molecular nitrogen conversion on its volume rate in the temperature range of 773-873 K. It is clearly observed that the quantity of fixed nitrogen per injected molecular nitrogen increases to some extent with the amount of injected molecular nitrogen. A further increase of raw material rate does not change the level of nitrogen fixation. Despite shorter contact time, the increase of N2 volume rate from 1 to 4 l/h intensifies the reaction. The aqueous hydroperoxide rate is constant for all nitrogen rates. However, at nitrogen rate of 4-5 l/h the fixed nitrogen yield is altered. Thus, chemical induction is the main factor promoting N2 fixation in N2-H2O2-H2O system. It manifests itself owing to coupling of H2O2 dissociation reactions, which generate the intermediate-HO2 radical-to the system. This intermediate transfers the induction action of the primary reaction to N2 oxidation process. In this case, H2O2 is injected in amounts much greater than demanded by N2 oxidation, because the main requirement for effective chemical coupling is the presence of HO2 in high concentration. To conclude the discussion, it should be noted that oxidative fixation of molecular nitrogen with hydrogen peroxide is rather simple for process engineering design, usually proceeds under homogeneous conditions without any catalyst under atmospheric pressure, and produces high yield of fixed nitrogen.
Kinetics of nitrogen fixation with hydrogen peroxide: molar ratio N2:30% H2O2 = 1:1.6; 1 – 3 – T = 773, 823 and 873 K, respectively; 4 – 6 – theoretical curves at these temperatures