Alain Tressaud
ICMCB-CNRS, University Bordeaux, France
Title: Fluorine, a key-element for the XXIst century: Uses of fluoride materials in modern technologies
Biography
Biography: Alain Tressaud
Abstract
Inorganic fluorine based compounds can be found as components in many applications, including energy storage and conversion, microphotonics, fluorescent chemical sensors, solid state lasers, nonlinear optics, nuclear cycle, superhydrophobic coatings, etc. Most of these outstanding properties can be correlated to the exceptional electronic properties of the fluorine element, yielding almost unique types of bonding with the other elements. The strategic importance of inorganic fluoride materials will be illustrated by several examples taken from various scientific domains: 1) In the field of energy storage, fluorinated carbon nano-particles (F-CNPs) have been tested as active materials in primary lithium batteries. In secondary Li batteries, 3d-transition metal fluorides and oxyfluorides mostly based on iron and titanium have been proposed as electrodes. 2) Nanocrystalline metal fluorides derived from fluorite (CaF2) or tysonite (LaF3) types exhibit high F- anionic conductivity that can be used as solid electrolytes in F- ion based all solid state batteries. 3) Upconversion and luminescent phenomena in nano fluorides based on rare earth metals. 4) Transparent fluoride ceramics which may challenge single crystals or glasses for solid state lasers applications. 5) Among transparent conductive oxides and oxide-fluorides, F-doped SnO2 exhibits rather good transparency in the visible range and high infrared absorption associated to its conductivity due to n-type charge carriers. 6) Perovskite related solid state fluorides based on d-transition metals exhibit a huge variety of structural and magnetic behaviors. Layered BaMF4 and iron fluorides (TTB- K3Fe5F15) are important families of multiferroics, in which magnetism and ferroelectricity coexist. 7) Intercalation of fluorine in several networks of oxides allows tuning the transition metal oxidation state. Superconductivity was created in F-doped cuprate systems La2CuO4 and Sr2CuO3 using low-temperature fluorination by F2 gas, or in F-doped oxypnictide LnFePnO1-xFx (Tc ~58 K). Finally, functionalization processes using various fluorination treatments yield nanosized materials, high surface area fluorides, or switchable hydrophobic/hydrophilic coatings.
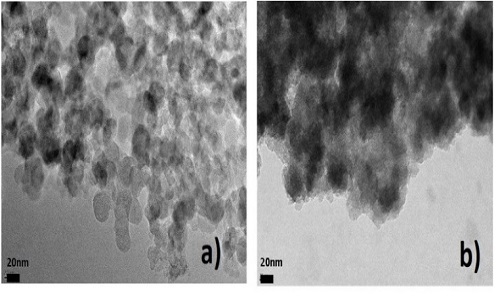
The nanosize of F-CNPs obtained from nano-carbons prepared in molten carbonates (b), is not changed after low-temperature F2 fluorination (a)