Zolaikha Rasouli
University of Kurdistan, Iran
Title: A joint spectrochemical, chemometrics and DFT/TD-DFT analysis of the ZnII, CuII and FeII metal ions complexation by MTB in aqueous solution
Biography
Biography: Zolaikha Rasouli
Abstract
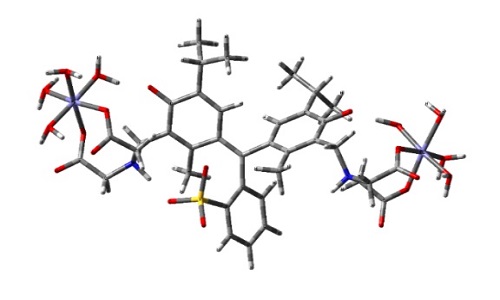
Over the past few years quantitative determinations of micro/macro minerals have attracted considerable attention. This attention is because of the essential role of minerals in various procedures i.e., medical, food, industrial and their numerous biological activities on human health. Chelation of chromomeric agents to metallic ions is one of the most efficient practices used in quantitative determination of micro/macro minerals in recent year. Herein, complexation systems formed by methylthymol blue (MTB) and ZnII, CuII and FeII metal ions in an aqueous solution with pH=5.0 have been described from experimental and theoretical points of view. It was characterized using UV-Vis absorption spectroscopy combined with soft/hard chemometrics methods and time dependent density functional theory (DFT/TD-DFT) calculations. First, an exploratory analysis from the acid-base system of MTB and each of the complex formation systems was carried out by Marquardt-Levenberg, MCR-ALS and RAFA algorithms. The results revealed that ZnII and CuII have the same behavior in confronting with MTB, both 1:2 and 1:1 stoichiometries of ZnII or CuII to MTB. However, for FeII, simultaneous formation of FeL and Fe2L complexes were suggested. In the second step of our work, we were interested to the description in detail of these systems by DFT/TD-DFT calculations to identify the nature of their structural geometries in the aqueous solution. For this purpose, we proposed the molecular hypothetical structures for different complex species based on the favorably moieties included in the complexation reactions, the protonation state of different functional groups of MTB and finally, the metal ion surrounding.
Molecular structure of Fe2L complex