Quirina Roode Gutzmer
Freiberg University of Mining and Technology, Germany
Title: Scaling up the synthesis of a hydroxyquinoline functionalized calix[4]arene – A designer molecule for selective rare earth extraction
Biography
Biography: Quirina Roode Gutzmer
Abstract
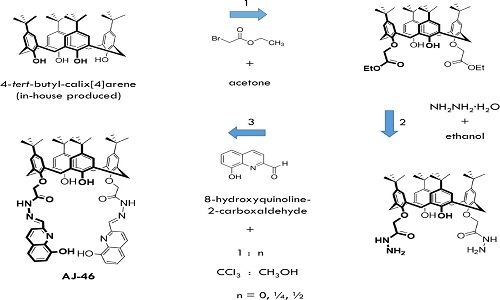
Currently the global consumption for rare earths for the production of permanent magnets, catalysts and luminescent materials, is steadily increasing. This will necessitate a recycling strategy in the future. Within the framework of the SE-FLECX project, several calix[4]arenes were developed with the objective of separating f-elements from other elements in aqueous solutions arising from ore leachates. Another aim is to remove actinides from such solutions. The most promising calix[4]arene that emerged from this work is a hydroxyquinoline functionalized calix[4]arene (AJ-46): 5,11,17,23-Tetra-p-tert-butyl-25,27-bis[(8-hydroxyquinolinemethine- hydrazinocarbonyl)-methoxy]-26,28-dihydroxycalix[4]arene. This calixarene demonstrates selectivity between light and heavy lanthanides in a solvent extraction process, as well as between actinides and lanthanides, thereby enabling radioactive decontamination. The objective of the work presented in this poster is to scale up the synthesis of AJ-46 so that a pilot-scale mixer-settler solvent extraction operation can be implemented using real ore leachates. The laboratory scale synthesis as developed by Jäschke et al. is not directly translatable for a technical production. The synthesis scheme achieved is shown in figure 1. In the first synthesis stage, acetonitrile was successfully replaced by acetone in a significantly reduced volume than used by Collins et al. Despite containing residual acetic acid and ethyl acetate, the 4-tert-butyl-calix[4]arene produced in-house according to Gutsche and Iqbal, produced a diester product in similar yield (79%) and quality after re-crystallization in a dichloromethane/ethanol solution (1:5) at -30℃ over 3 days. In the third synthesis stage, the solvent volume could be reduced 5-fold and was accomplished by replacing ethanol with a 3:1 ethanol/chloroform solvent mixture. The final product is obtained in a yield of 88-91%.
Scheme for the scale-up synthesis of the hydroxyquinoline functionalized calix[4]arene