Ayrat R Khamatgalimov
Arbuzov Institute of Organic and Physical Chemistry - FRC Kazan Scientific Center of RAS, Russia
Title: Phenalenyl like substructures in fullerene molecules
Biography
Biography: Ayrat R Khamatgalimov
Abstract
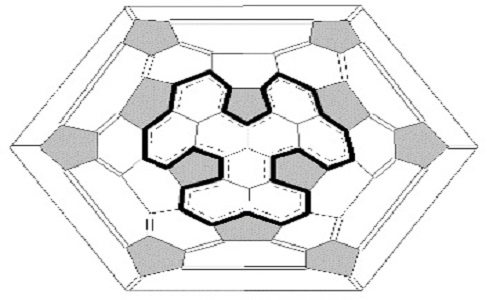
In our research we are developing an alternative approach in which a fullerene molecule is considered to contain a number of substructures incorporated in the fullerene shell and resembling aromatic analogues such as indacene, pyrene, perylene, corannulene, coronene, phenalenyl radical, etc. Our result shows that the presence of phenalenyl-radical substructures with unpaired electron leads to instability of whole fullerene molecule. For example, fullerene C74 is unstable due to the presence of two phenalenyl-radical substructures, four such radical substructures caused of instability of fullerene C76 too. Here we report the computational characterizations of non-planar polycyclic hydrocarbon species that can be cut from fullerene molecule – substructure from three symmetrically fused phenalenyl composed by nine fused benzenoid rings. The results of quantum-chemical calculations show that this structure has an open-shell ground state and a relatively small HOMO–LUMO gap. This is due to the fact that this molecule is derivative of the phenalenyl-radical. Analysis of row of isolated-pentagon-rule (IPR) isomers of higher fullerenes from C72 to C104 shows that this substructure is present in structure of some of them, for example, in IPR isomer 7 (C3v) of C82 fullerene. Indeed, our researches show that this molecule has an open-shell structure due to containing radical substructures (like phenalenyl-radical substructure). It should be noted that spin densities in triplet configuration of isomer 7 (C3v) of C82 fullerene are mainly concentrated namely on radical substructures likewise the C74 biradical. Thus, our results shows that such fullerenes are unstable and can`t be obtained as empty molecules. However they become stable as exohedral or endohedral derivatives or in polymeric forms.
Schlegel diagram of isomer 7 (C3v) of C82 fullerene with depicted phenalenyl-like substructure