Takeyuki Suzuki
Osaka University, Japan
Title: Ir catalyzed asymmetric tandem reaction of meso-diols Ir catalyzed asymmetric tandem reaction of meso-diols
Biography
Biography: Takeyuki Suzuki
Abstract
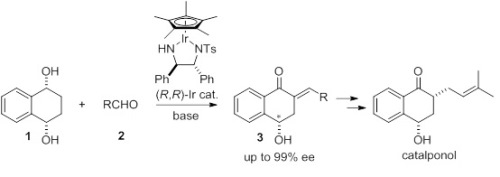
The development of tandem catalytic processes is one of the main subjects for the modern synthetic organic chemistry. Recently we have reported oxidative desymmetrization of meso-diols using chiral iridium complex catalysts. The reaction is safe and environmentally benign process without use of stoichiometric amount of heavy metals. We report here that asymmetric tandem coupling of meso-diols and an aldehyde using a chiral Ir catalyst. This tandem reaction consists of oxidative desymmetrization of meso-diols, aldol condensation with an aldehyde. The reaction of meso-diol1, benzaldehyde2 in the presence of a chiral Ir complex (10 mol %) and CsOH in THF gave the desired benzylidene ketone3 in 82% yield with 96% ee. With the efficient multi-catalytic oxidative desymmetrization of meso-diols in hands, we applied this methodology to the synthesis of catalponol. The corresponding dienone was obtained in 87% yield with 99% ee. Finally, catalponol was synthesized by the regio- and stereoselctive reduction in good yield.
References: